Developing a Superior Zinc Anode for Aqueous Secondary Batteries
I worked as a research assistant in the
Chen Lab at in Hong Kong University of Science and Technology during my gap year between August 2020 - May 2021.
One of my primary research focus was on developing high performance zinc batteries using a novel phenomenon called reduction-induced decomposition .
In the world of increasing electrification, alternatives to lithium ion batteries draws an increasing amount of academic and commercial interests.
Zinc, as the most abundant metal on Earth, offers a high theoretical capacity (820 mAh/g) without the toxicity and safety risks inherent with lithium based batteries.
However, many technical challenges remain unsolved in order to increase the performance of zinc-based aqueous batteries to practical engineering standards.
On the electrochemical front, two dominating failure mechanisms, dentritic growth and shape change, have continued to plague the development of zinc based batteries for the past 30 years. To overcome these challenges, the porous zinc metal electrode needs to maintain the electrically conductive Zn 'network' under high depths of discharge.
On the electrochemical front, two dominating failure mechanisms, dentritic growth and shape change, have continued to plague the development of zinc based batteries for the past 30 years. To overcome these challenges, the porous zinc metal electrode needs to maintain the electrically conductive Zn 'network' under high depths of discharge.
Importance of a Bicontinuous Structure
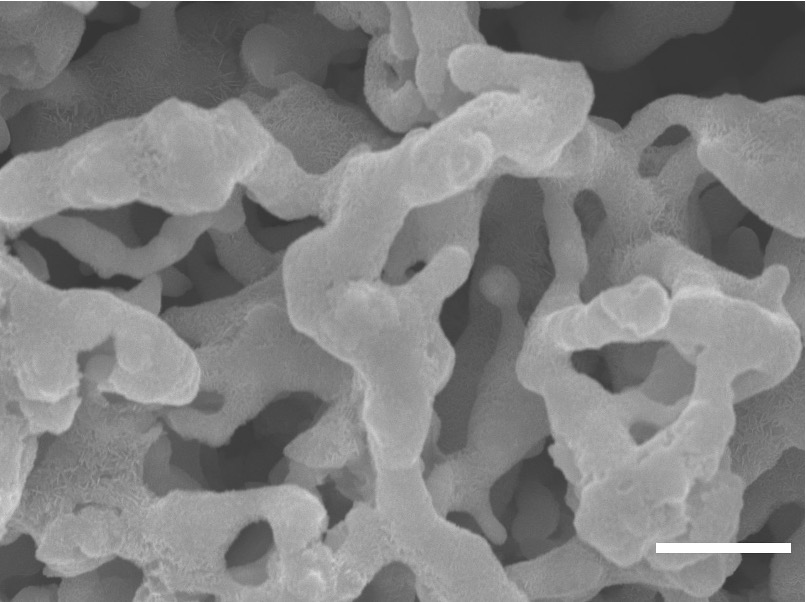
To achieve an optimal electrode structure, we designed a new simple process to electrochemically reduce a ZnO powder precursor into a bicontinuous metal zinc anode,
very similar to structures derived from a monlithic salt precursor.
The current reduction pathway is a two step process illustrated in the equations below:
\[ZnO + H_2O + 2OH^{-} → Zn(OH)_4^{2-}\] \[Zn(OH)_4^{2-} + 2e^{-} → Zn + 4OH^{-}\]
Developing this fabrication method was one of my primary tasks of the project.
To combat shape change of the active material macro redistribution through zincate transport in bulk electrolyte, it is important that the zincate reduction be much faster than the first step.
Though similar to deep charging a typical Zn||NiOOH battery, which typically uses a ZnO powder electrode, there are a couple differences.
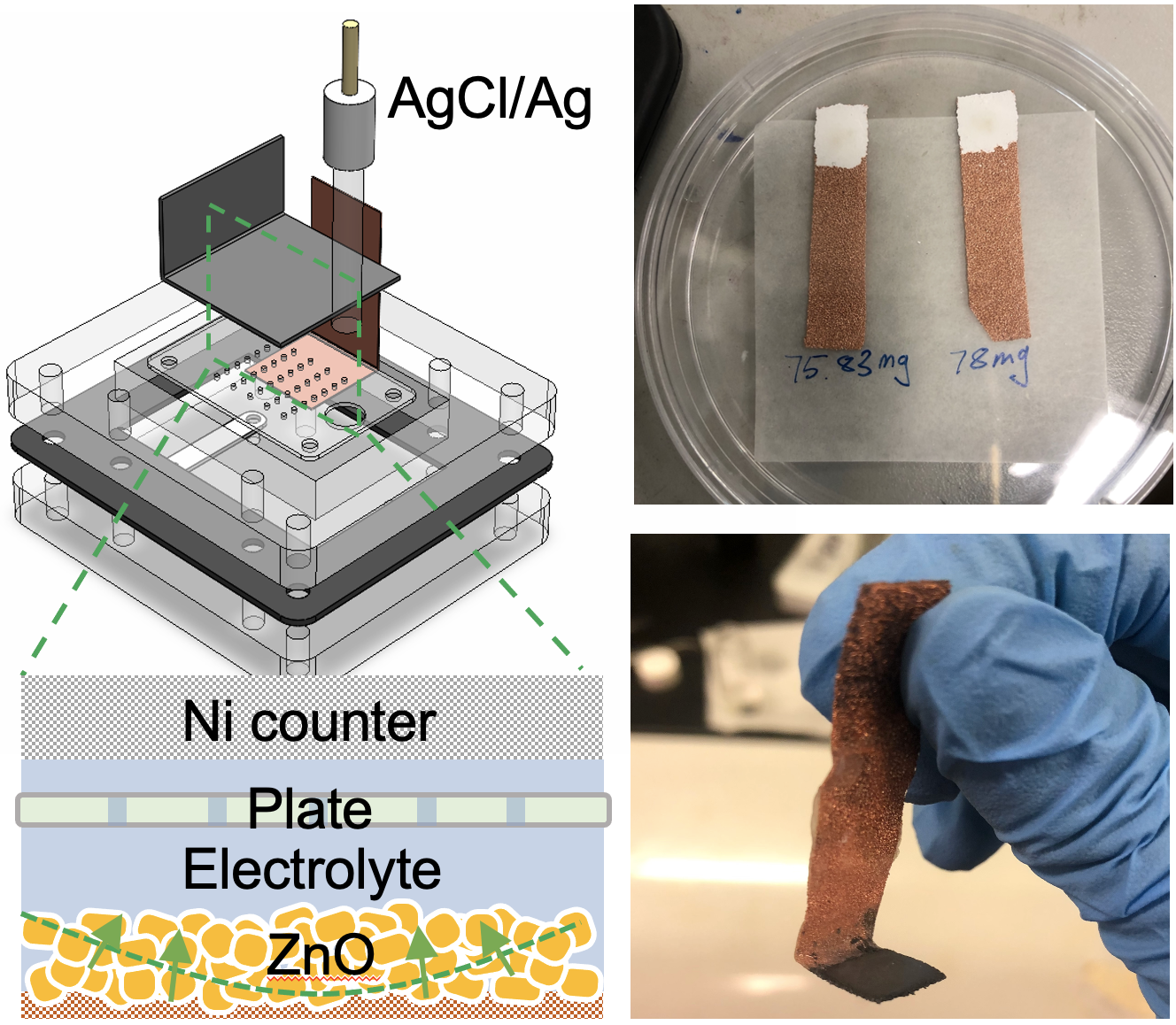
By using a much lower concentration of electrolyte (3M KOH) and a higher over potential in a custom electrochemical cell, we promoted a uniform reaction and reduce >95% of all ZnO into the bicontinuous zinc structures.
In addition, I performed 2D erosion analysis on binarized SEM images of the nonporous Zn anode and models of Zn powder compact anode. The results from this simple numerical model demonstrated how our unique bicontinous structure maintained connectivity at high depths of discharge.
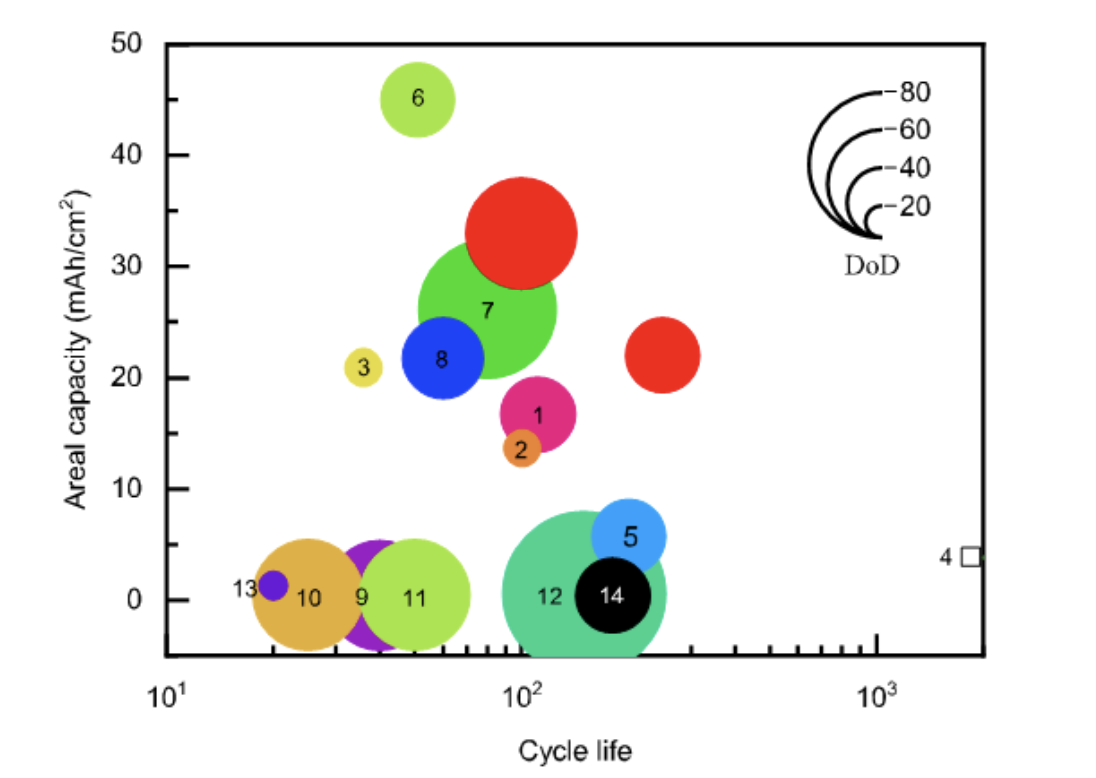
To better understand our electrochemical performance, we can benchmark our areal capacity, cycle life, and depth of discharge in Zn||NIOOH coin cells against other state of the art zinc electrodes reported in literature.
Even with realistic charge/discharge testing parameters in a lean electrolyte configuration, we were able to demonstrate state of the art performance.